Physical Change Particle Diagram
Matter particle model kinetic molecular simple states particles liquids solids gases state energy different changes move science Physical change state sublimation deposition chemistry changes cycle gas materials ice solid matter diagram examples water chemical science three between 3. particle model of matter
Science online: What is the difference between the physical changes and
Change physical particle model understanding science bitesize bbc 7.2 understanding physical change Kinetic melts molecules socratic besides
The particulate nature of matter
Matter particulate liquid diagram igcse evaporation summaryTransition flowchart Matter changes states energy gases liquids solids state change solid particle particles model melting when thermal temperature move weebly liquidThe particle model – gcse chemistry (combined science) ocr revision.
Matter particle solid liquid gas theory gases kinetic model energy science particles movement states bbc gcse physics revision chemistry moleculeSavvy-chemist: gcse ocr gateway chemistry c1.1 the particle model Science online: what is the difference between the physical changes andWhat happens to the kinetic energy of its molecules as ice melts into.

Use of particulate-level instruction on chemical and physical changes
3. particle model of matterParticle particles gcse chemistry kinetic temperature increase stored increases 33 label each transition in this flowchart as a chemical change or aChange physical changes believe chemical particulate level matter states instruction use chemedx.
.


The Particle Model – GCSE Chemistry (Combined Science) OCR Revision

Use of Particulate-Level Instruction on Chemical and Physical Changes
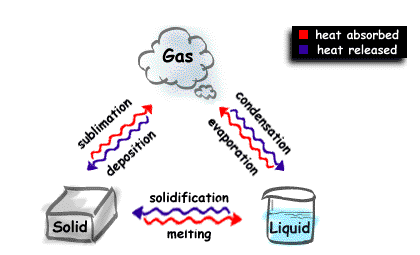
Science online: What is the difference between the physical changes and

The Particulate Nature Of Matter - IGCSE Chemistry | Free Exam Academy

3. Particle Model of Matter - THOMAS TALLIS SCIENCE

7.2 Understanding Physical Change - Year 8 Science

3. Particle Model of Matter - THOMAS TALLIS SCIENCE

What happens to the kinetic energy of its molecules as ice melts into